Mössbauer spectroscopy
by Sylvia-Monique Thomas, University of Nevada Las Vegas
Outline
Introduction
Theory
Instrumentation
Applications
Guest Lecturer
Literature
Related Links
Acknowledgements
Introduction

What is Mössbauer spectroscopy?
Mössbauer spectroscopy, named after the german physicist Rudolf Mössbauer, is used to study the local atomic environment around nuclei in a solid and thus provides information at the atomic level. In mineralogy Mössbauer spectroscopy is used to determine redox ratios, spin and valence state, and the site occupation (coordination polyhedra) of atoms in the crystal structure.
A calendar of the history of Mössbauer spectroscopy was put together by Prof. em. Leopold May of the Catholic University of America.
Advantages
· high energy resolution
· nuclide specific
· high nuclear cross section
· short range probe, allows studies of amorphous or poorly crystalline material
· in-situ studies at high P and/or T are possibleTheory
The Mössbauer effect
The Mössbauer effect describes the recoilless emission and resonant absorption of gamma radiation by atomic nuclei in solids [16].
If an atomic nucleus emits energy, in this case a γ quantum in a gas or a fluid the γ quantum looses the recoil energy ER. This recoil energy is then transmitted back to the emitting nucleus for energy preservation. To enable absorption of the γ quantum by other atomic nuclei in a gas or liquid the energy of the γ quantum has to be increased by ER. This is why recoilless emission and absorption in a gas or liquid are not possible.
In a solid, however, the recoil energy may be annihilated (ER⇨0) and recoil-free emission and absorption become possible. There is a certain portion f of the emitting nuclei, the so-called recoil-free fraction (Debye-Waller factor, Lamb-Mössbauer factor), which is high enough in solids so γ rays can be emitted without loss of energy of the γ quantum. F becomes larger with decreasing Eγ, the recoil-free energy of the γ quantum, and decreasing χ2, the mean square vibrational amplitude of the emitting nucleus in the direction of the γ ray. Another important parameter is the line width of the gamma ray, which depends on the lifetime of the nuclear excited state. A small line width allows the detection of small changes of the γ radiation energy, i.e. the detection of nuclear hyperfine interactions.
Hyperfine parameters
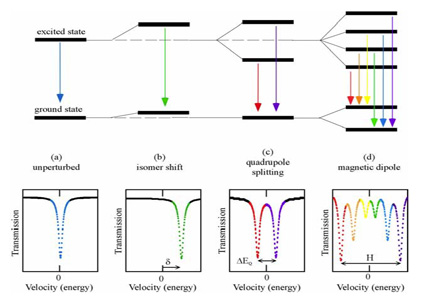
1) Isomer shift (chemical shift)
The total isomer shift (δ) or centre shift δ consists of δ0 and δSOD. It depends on coordination, valence state and spin state of atoms and thus on s electron density and p and d electrons.
This shift of nuclear levels is caused by anelectrostatic interaction between the positively charged nucleus and the negative electronic charge . It is called isomer shift because it depends on the difference in the nuclear radii of the ground (g) and isomeric excited (e) states.
In Mössbauer spectroscopy the nuclear energy of the γ ray transitioning between two nuclear levels in the source is compared with that in the absorber, which differ because of different electronic and crystallographic environments. Energy differences as small as 10-10 eV can be measured and δ0 is calculated relative to a standard (e.g., for iron: isomer shift δ0 of α-iron at room temperature). Using the difference in isomer shift δ0 (i.e. distance between centroids of the doublets and the zero point of the velocity scale) for example Fe2+ and Fe3+ can be distinguished in a mineral. For Mössbauer nuclei, which have a smaller radius in the excited state than in the ground state, such as 57Fe, the isomer shift δ0 decreases with increasing electron density at the 57Fe nucleus due to increasing valence or covalent bonding. For Mössbauer nuclei, which have a larger radius in the excited state than in the ground state, such as 119Sn, an increasing electron density leads to an increase of the isomer shift.
A second-order Doppler shift δSOD causes the temperature dependence of the isomer shift. δSOD depends on the force constant K and the mean square displacement χ2 of the nucleus. As χ2 increases with temperature, δSOD increases and the total isomer shift δ decreases.
2) Quadrupole splitting (QS or ΔEQ)
The quadrupole splitting is a measure of the degree of crystallographic site distortion, the valence, spin state and coordination of the absorber atoms.
An electric quadrupole interaction, the splitting of nuclear energy levels, is caused by the interaction between the nuclear quadrupole moment Q (nuclear spin quantum number I > 1/2) and the non-cubic component of its surrounding electric field. In the case of 57Fe the excited state of the 57Fe nucleus is split, thus two nuclear transitions from the ground state to the excited states rather than just one occur. This results in two corresponding peaks in the Mössbauer spectrum. The energy difference between the two peaks describes the energy difference between the two excited states, i.e. the quadrupole splitting. The magnitude of the QS depends on the electric field gradient (EFG) q. The EFG is non-zero if the charge distribution around the nucleus (valence electrons and lattice ions) deviates from cubic symmetry.
3) Magnetic hyperfine splitting (ΔEM)
A nucleus with a spin quantum number I > 1 has a non-zero magnetic dipole moment. Theinteraction of this nuclear magnetic dipole moment µ and a magnetic field H (internal or external) at the nucleus causes magnetic hyperfine splitting. In the case of 57Fe the excited state (I =3/2) is split into 4 sublevels and the ground state (I=1/2) is split into 2 sublevels. This results in a peak sextet in the Mössbauer spectrum.
Instrumentation
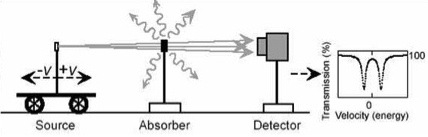
A linear geometry, with the detector arranged behind the source and absorber, is used for transmission studies to study all nuclei in the sample. A backscatter geometry in contrast can be used to study surface nuclei of the sample. The latter technique is called Conversion Electron Mössbauer Spectroscopy (CEMS) [15].
The source is a radioactive parent of a Mössbauer isotope, e.g. 57Co is the parent of 57Fe. An ideal Mössbauer source has a relatively low energy of the gamma radiation Eγ, thus a high recoil free fraction f, a long mean lifetime t of the first excited nuclear level, and thus a small half width G.
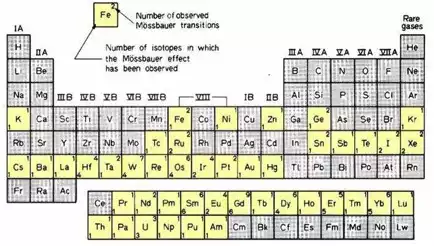
A popular Mössbauer isotope is iron, because the half-life of its parent 57Co is reasonable long (270 days), the natural line width is relatively small (0.097 mm/s), recoil-free fractions are generally high at room temperature and the absorption cross section is sufficiently high that reasonable spectra can be obtained even for absorbers with low iron concentrations. The source diameter for conventional 57Co sources is in the order of 1 cm. It depends on the density of the parent nuclei and their number required for a sufficient count rate. Other suitable Mössbauer isotopes, such as 119Sn and 121Sb are highlighted in the periodic table.
The absorber, the sample, can be a powder, glass, single crystal, a melt or a liquid. The sample can be cooled, heated, measured under high-pressure or in an external magnetic field.
The detector can be a gas-proportional counter, a multi-channel analyzer combined with scintillation or semiconductor detectors, or a resonance counter.
Applications
· Mössbauer spectroscopy and synchrotron radiation
· Mössbauer spectroscopy and single-crystals in diamond anvil cells
Guest Lecturer
Literature
Review articles:
1) Amthauer et al. (2004) Mössbauer spectroscopy: Basic principles. In: Beran, A. & Libowitzky, E. (eds.) Spectroscopic Methods in Mineralogy. EMU Notes in Mineralogy, 6, 345-367.
2) Hawthorne, F.C. (1988) Mössbauer spectroscopy. In: Hawthorne, F.C. (ed.): Spectroscopic methods in mineralogy and geology. Reviews in Mineralogy, 18, Washington, D.C.: Mineralogical Society of America, 255-340.
3) McCammon, C. (2004) Mössbauer spectroscopy: Applications. In: Beran, A. & Libowitzky, E. (eds.) Spectroscopic Methods in Mineralogy. EMU Notes in Mineralogy, 6, 369-398.
Textbooks:
4) Bancroft, G.M. (1973) Mössbauer spectroscopy. An introduction for inorganic chemists and geochemists. New York (N.Y.): McGraw-Hill. ...
5) Dickson, D.P.E. and Berry, J.F (1986) Mössbauer spectroscopy. Cambridge University Press, 274 pp.
6) Gerdau, E. & de Waard, H. (1999) Nuclear resonant scattering of synchrotron radiation / Hyper. Inter. Spec. Issues, 123-124.
7) Gonser, U. (ed.) (1975) Mössbauer spectroscopy. Berlin: Springer-Verlag.
8) Greenwood, N.N. & Gibb, T.C. (1971) Mössbauer spectroscopy. London: Chapman & Hall, 659 pp.
9) Guetlich, Pl., Link, R. & Trautwein, A. (1978) Mössbauer spectroscopy and transition metal chemistry. Berlin: Springer Verlag.
10) Long, G.J. (ed.) (1984, 1987, 1989) Mössbauer spectroscopy applied to inorganic chemistry. Volumes 1-3, New York (N.Y.): Plenum Press.
11) Long, G.J. & Grandjean, F. (eds.) (1996) Mössbauer spectroscopy applied to magnetism and material science, vol. 2, Mod. Inorg. Chem. Ser./. New York (N.Y.): Plenum Press.
12) Marfunin, A.S. (1979) Spectroscopy, luminescence and radiation centers in minerals. Berlin: Springer-Verlag, 352 pp.
13) Shenoy, G.K. & Wagner, F.E. (1978) Mössbauer isomer shifts. Amsterdam: North Holland.
14) Wertheim, G.K. (1964) Mössbauer effect: Principles and applications. New York (N.Y.) Academic Press.
Applications:
15) Nomura, K. (1999) Conversion electron Mössbauer spectrometry. In Miglierini, M. & Petredis, D. (eds.): Mössbauer spectroscopy in material science. Dordrecht: Kluwer, 63-78.
Historic:
16) Mössbauer, R. L. (1958). "Kernresonanzfluoreszenz von Gammastrahlung in Ir191" (in German). Zeitschrift fur Physik A, 151 (2): 124–143.
17) Mössbauer, R. L. (1961) Recoilless nuclear resonance absorption of gamma radiation. Nobel lecture, December 11th 1961.
18) The Rudolf Mössbauer Story
Related Links
· Mössbauer spectroscopy by M. Darby Dyar site
· Mössbauer interactiv periodic table
Tutorials:
· Mössbauer spectroscopy: A powerful tool in scientific research
· Introduction to Mössbauer spectroscopy Parts 1-4
Databases:
· Mössbauer effect data center ( This site may be offline. )
Acknowledgements
These materials are being developed with the support of COMPRES, the Consortium for Materials Properties Research in Earth Sciences, under NSF Cooperative Agreement EAR 10-43050 and is partially supported by UNLV's High Pressure Science and Engineering Center, a DOE NNSA Center of Excellence supported under DOE NNSA Cooperative Agreement No. DE FC52-06NA26274.

